The information on this page is archived and provided for reference purposes only.

DEPARTMENT OF HEAlTH AND HUMAN SERVICES
Public Health Service
National Institutes of Health
National Cancer Institute
National Institute of Environmental Health Sciences
REPORT TO THE U.S. CONGRESS
THE NORTHEAST AND MID-ATLANTIC
BREAST CANCER STUDY (NE/MA)
July 2000
Table of Contents
STUDY PARTICIPANTS
EXECUTIVE SUMMARY
INTRODUCTION
PART I
BACKGROUND
- Epidemiology of Breast Cancer
- Questions Leading to NE/MA
- History and Description of NE/MA Projects
- Collaborations
PART II.
RESULTS
- Abstracts
Environmental and Genetic Determinants of Breast Cancer Environmental Factors and Breast Cancer Risk in Maryland Environmental Risk Factors and Breast Cancer in the Nurses' Health Study EMF and Breast Cancer on Long Island Study Environmental and Genetic Determinants of Breast Cancer Organochlorine Compounds and Risk of Breast Cancer - Summary
PART III.
CONCLUSIONS
REFERENCES
Appendix A: Cancer Registries Amendment Act, 1992 Senate Appropriations Subcommittee Report for NIH, fiscal year 1993
Appendix B: RFA CA/ES-93-024
Appendix C: Funding Table
Appendix D: NCI Press Release "The Northeast/Mid-Atlantic Study," 1994
Appendix E: Representative NE/MA Questions (Interested individuals should contact the investigators)
Appendix F: Publications
Appendix G: Copies of publications
THE NORTHEAST AND MID-ATLANTIC BREAST CANCER STUDY
Principal Investigators
Jo L. Freudenheim, Ph.D.
Department of Social and Preventive Medicine
State University of New York at Buffalo
Buffalo, New York
Kathy J. Helzlsouer, M.D., M.H.S.
Department of Epidemiology
School of Hygiene and Public Health
Johns Hopkins University
Baltimore, Maryland
David J. Hunter, M.B.B.S., Sc.D.
Brigham and Women's Hospital
Channing Laboratory
Harvard School of Public Health
Boston, Massachusetts
M. Cristina Leske, M.D., M.P.H.
Department of Preventive Medicine, Division of Epidemiology
University Medical Center at
Stony Brook Stony Brook, New York
Mary S. Wolff, Ph.D.
Department of Community Medicine
Mount Sinai School of Medicine
New York, New York
Tongzhang Zheng, M.D., Sc.D.
Department of Epidemiology and Public Health
School of Medicine
Yale University
New Haven, Connecticut
National Institutes of Health Staff
Kumiko Iwamoto, M.D., Dr.P.H.
Program Director
Division of Cancer Control and Population Sciences
National Cancer Institute
Gwen Collman, Ph.D.
Program Director
Division of Extramural Research and Training
National Institute of Environmental Health Sciences
G. Iris Obrams, M.D., Ph.D.
Associate Director
Epidemiology and Genomics Research Program
Division of Cancer Control and Population Sciences
National Cancer Institute
Institute Directors
Richard D. Klausner, M.D.
Director
National Cancer Institute
Kenneth Olden, Ph.D.
Director
National Institute of Environmental Health Sciences
EXECUTIVE SUMMARY
In response to a request of the 1992 Senate Appropriations Committee (Appendix A: Senate Report 102-397, page 76, and the Cancer Registries Amendment Act, P.L. 102-515), the National Cancer Institute (NCI) assumed the lead responsibility at the National Institutes of Health and was joined by the National Institute of Environmental Health Sciences (NIEHS) to fund a study on factors that may contribute to the high breast cancer mortality rates in the northeastern and mid-Atlantic regions of the United States. In accordance with the Committee's request to allocate $1 million beginning in fiscal year 1993, the NCI and the NIEHS have provided a total of $8,748,621 to support the 5-year research effort.
Questions regarding the health impact of environmental substances are not new, and seeking answers is a formidable task. Several Federal agencies support activities that are building scientific databases for use in environmental health research. A major hurdle is estimating previous exposures to noxious environmental pollutants, particularly on an individual level. Often, even if known, substances of interest may not be present in detectable or pure concentrations. Although epidemiologic studies can provide clues to suspected causes of cancer, the classical example being tobacco and lung cancer, confirmation of findings and large study populations are required. Additionally, identification of an environmental carcinogen (cancer-causing substance) is especially challenging because cancer is a disease that may take years to develop after the impact of multiple factors. In fulfilling the Congressional directive within the context of the research limitations described, the six collaborating projects of the Northeast and Mid-Atlantic Breast Cancer Study (NE/MA) applied the best technology and study methods available.
There was concurrence among projects in observing no association between breast cancer risk and blood levels of the organochlorine compounds, DDT pesticide and its metabolite, dichloro-2,2-bis (p-chlorophenyl) ethylene (DDE) and polychlorinated biphenyls (PCBs). Some projects analyzing small subgroups of women found that certain characteristics (e.g., menopausal status, cigarette smoking, breastfeeding, having genotypes related to carcinogen-metabolizing enzymes, vitamin B-12 serum levels) differed in women with breast cancer as compared with those without breast cancer. These analyses provide intriguing clues for future research. Readers are referred to Part II, Results, where more detailed explanations of significant findings are presented.
In summary, NE/MA is unique for conducting concurrent epidemiologic studies of breast cancer and the environment and focusing on one region of the United States. Because there have been conflicting reports in the literature regarding the relationship between organochlorines and breast cancer, the consistency in NE/MA results is informative, and findings provide a knowledge base for directing future investigations.
INTRODUCTION
The Senate Appropriations Committee mandated the Northeast and Mid-Atlantic Breast Cancer Study (NE/MA) in the Cancer Registries Amendment Act, P.L. 102-515 (Appendix A) in 1992 and included the following language in the fiscal year 1993 Senate Appropriations Subcommittee Report for the National Institutes of Health (NIH) (Appendix A):
"The Committee is concerned by the high breast cancer mortality rates in the northeastern and mid-Atlantic regions of the country and directs the National Cancer Institute to conduct a study with updates for 4 succeeding years for the purpose of determining the factors contributing to the high breast cancer mortality rates in Connecticut, Delaware, Maryland, Massachusetts, New Hampshire, New Jersey, New York, Rhode Island, Vermont, and the District of Columbia. The NCI is directed to develop a plan for conducting the study and shall provide a copy of such plan to the House and Senate Committees on Appropriations and to the Committee on Labor and Human Resources of the Senate by July 1, 1993. The Committee expects that $1 million will be made available for fiscal year 1993 for this study."
In response to this directive, the National Cancer Institute (NCI) and the National Institute of Environmental Health Sciences (NIEHS) issued a competitive request for applications entitled "Environmental Factors and Breast Cancer in High-Risk Areas" (Appendix B). After due process in the NIH peer review system, grant support was awarded to investigator-initiated proposals ranked for scientific excellence and responsiveness to the Congressional mandate.
The NE/MA, comprised of six independently funded and collaborating institutions, was funded by the NCI and NIEHS in September 1993. The research objective of NE/MA was to evaluate measurable environmental exposures in association with known breast cancer risk factors that could contribute to or be associated with the high occurrence of female breast cancer in the northeastern and mid-Atlantic regions of the United States.
The report is organized in four sections. The Introduction states the Congressional directive for conducting NE/MA and describes the response from the NCI. Part I, the Background , presents an overview of breast cancer relevant to NE/MA and describes the history and components of NE/MA. A table summarizing the funds received by each institution in NE/MA is included in Appendix C. Part II, Results , begins with abstracts from the six principal investigators (project leaders) stating major and significant findings of each project followed by a summary that consolidates the results. The Conclusions , Part III, discusses implications of NE/MA results and explores future research directions.
PART I. BACKGROUND
A. Epidemiology of Breast Cancer
Breast cancer is the most frequently diagnosed nonskin cancer among women in the United States. It is second only to lung cancer in cancer-related deaths. Approximately 175,000 new cases of breast cancer were estimated to be diagnosed in 1999 with about 43,300 women expected to die from the disease.1 Mortality rates from breast cancer have declined between 1988 and 1996, but there are disparities within these data. Younger women and white women have experienced greater reductions in mortality than African-American women and women age 65 and older.
Although most cases of breast cancer cannot be attributed to a specific cause, epidemiologic evidence has identified certain factors that are known to contribute to breast cancer development. To help inform the medical community and the public of these factors, the NCI released a press statement in July 1999 entitled Cancer Research: Because Lives Depend On It 2 that listed the following contributing factors for breast cancer.
- Age. The risk of developing breast cancer increases with age. The majority of breast cancer cases occur in women older than age 50.
- Family history. Having one or more first-degree blood relatives (sisters, mothers, daughters) diagnosed with breast cancer increases a woman's risk.
- Age at menarche. Women who had their first menstrual period before age 12 have a slightly increased risk of breast cancer.
- Age at first live birth. Women who had their first full-term pregnancy after age 30 and women who have never borne a child have a greater risk of developing breast cancer.
- Race. White women have a greater risk of developing breast cancer than black women (although black women diagnosed with breast cancer are more likely to die of the disease).
- Personal history of breast abnormalities. A change in breast tissue known as atypical hyperplasia diagnosed by breast biopsy or breast tissue abnormalities, ductal carcinoma in situ (DCIS), and lobular carcinoma in situ (LCIS), are associated with increased risk for developing breast cancer.
- Genes. An estimated 5 to 10 percent of all breast cancers can be attributed to genetic factors. Genes known as BRCA1 and BRCA2 are involved in about 3 to 4 percent of breast cancer cases. Other breast cancer-related genes are yet to be discovered.
Additional factors include hormone replacement therapy, alcohol consumption, and radiation exposure. Research is ongoing to evaluate the risks of diets high in fat and obesity in postmenopausal women, physical exercise (beneficial), and long-term breast feeding (protective).
Geographic variation in breast cancer rates has been well documented, both internationally and within the United States. According to the recently released Atlas of Cancer Mortality in the United States , 1950–94, 3 among white females, there have been continuing high rates in the northeastern and north-central regions and in scattered areas of the far western states with low rates in the South and Rocky Mountain areas (Figure 1). The north-south gradient in breast cancer risk also was observed between white males and black females, although not as pronounced as among white females. This geographic variation is predominantly observed in postmenopausal women. The differences in population distribution of known breast cancer risk factors such as menstrual and reproductive variables may account for 40 to 50 percent of breast cancer cases. 4
On the other hand, migrant studies have observed changes in breast cancer incidence in women who moved from low-risk countries to countries of higher risk, and the notable differences in international rates suggest that environment also may influence breast cancer development. Although public concerns have focused on exposure to environmental hazards such as pesticides, landfills, industrial chemicals, and electromagnetic fields, to date, there has been inconclusive evidence linking them to breast cancer.
B. Questions Leading to NE/MA
Noting the regional disparity in 1984–1988 breast cancer mortality rates in the northeastern and mid-Atlantic states (Table 1), Congress requested a region-specific study to explain the high rates. Very few results from animal experiments or human studies at that time pointed to likely research possibilities. Two preliminary reports of blood analyses in small groups of women 5 , 6 had suggested that certain pesticides in the environment might increase the risk of breast cancer. This observation seemed plausible due to earlier experimental, clinical, and epidemiologic data that associated hormones, particularly estrogen, with breast cancer risk, and because some pesticide compounds were known to be chemically similar to hormones.
The suspected pesticides, classified as organochlorine compounds, include dichloro-2,2-bis (p -chlorophenyl) ethylene (DDE), a metabolite of 1,1,1-trichloro-2,2-bis (p -chlorophenyl) ethane (DDT), and the polychlorinated biphenyls (PCBs). These chemicals are known as xeno- estrogens because they exert an estrogenic effect, though weak, on human tissue. 7 Because estrogenic activity is known to be associated with breast cancer risk, the focus on xeno-estrogens was thought to be a potential field of research that might yield conclusive results.
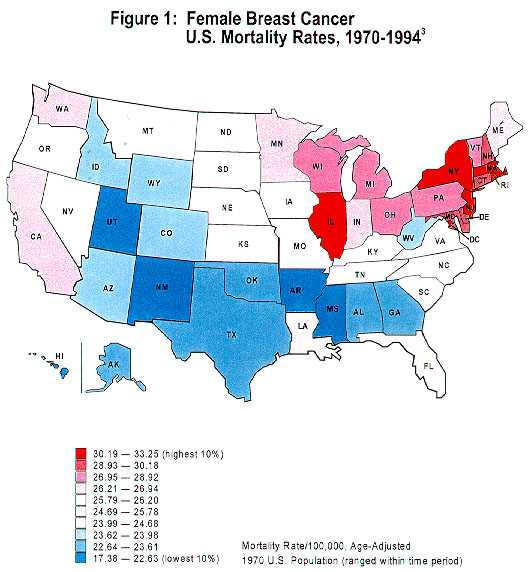
|
TABLE 1. U. S. Mortality Rates for Female Breast Cancer,* 1984–1988 and 1992–1996 (BOLD indicates NE/MA states) |
|||||
|---|---|---|---|---|---|
| State | 1984–88 | 1992–96 | State | 1984–88 | 1992–96 |
| Alabama | 24.83 | 23.15 | Montana | 24.57 | 24.64 |
| Alaska | 22.21 | 23.20 | Nebraska | 26.06 | 24.76 |
| Arizona | 24.34 | 22.25 | Nevada | 26.93 | 24.86 |
| Arkansas | 21.96 | 23.06 | New Hampshire | 30.91 | 27.54 |
| California | 27.28 | 24.41 | New Jersey | 31.80 | 28.37 |
| Colorado | 24.20 | 21.65 | New Mexico | 23.95 | 23.06 |
| Connecticut | 29.39 | 25.65 | New York | 31.46 | 28.19 |
| Delaware | 33.45 | 27.82 | North Carolina | 26.82 | 24.86 |
| District of Columbia | 33.28 | 32.78 | North Dakota | 25.77 | 24.45 |
| Florida | 25.46 | 24.47 | Ohio | 29.26 | 27.16 |
| Georgia | 25.36 | 23.99 | Oklahoma | 23.71 | 24.18 |
| Hawaii | 18.18 | 17.55 | Oregon | 26.60 | 23.99 |
| Idaho | 22.08 | 22.31 | Pennsylvania | 29.30 | 27.74 |
| Illinois | 29.31 | 27.51 | Rhode Island | 32.14 | 27.98 |
| Indiana | 27.25 | 25.85 | South Carolina | 25.27 | 24.43 |
| Iowa | 25.94 | 24.61 | South Dakota | 25.83 | 24.25 |
| Kansas | 25.22 | 23.41 | Tennessee | 24.99 | 25.34 |
| Kentucky | 25.54 | 24.82 | Texas | 22.50 | 23.53 |
| Louisiana | 24.69 | 26.37 | Utah | 22.34 | 20.84 |
| Maine | 26.82 | 25.33 | Vermont | 30.36 | 24.79 |
| Maryland | 29.92 | 27.33 | Virginia | 27.68 | 25.81 |
| Massachusetts | 32.07 | 27.87 | Washington | 26.74 | 24.06 |
| Michigan | 28.67 | 25.99 | West Virginia | 24.93 | 23.37 |
| Minnesota | 27.53 | 24.51 | Wisconsin | 27.70 | 24.83 |
| Mississippi | 22.75 | 23.76 | Wyoming | 23.86 | 24.03 |
| Missouri | 26.31 | 24.63 | |||
|
U.S.
|
27.36 | 25.41 | |||
* Rates are per 100,000 and age-adjusted to the 1970 standard.
Source: Cancer Statistics Branch, NCI, 1999.
DDT use began in the United States in the 1940s and was used widely until 1972 when it was banned. Although no longer used in the United States, the compound takes many years to disappear from the environment. The concern with DDT and its metabolite DDE grew when it was learned that they persist in the blood and accumulate in the human body, specifically in adipose (fat) tissue. Numerous studies have shown that DDT accumulates in breast tissue and that infants are exposed to it during lactation.8 It has not yet been determined whether DDT causes cancer in humans, although DDT was classified by the International Agency for Research on Cancer (IARC) as "possibly carcinogenic" based on animal studies.9
PCBs are a group of more than 200 compounds, called congeners, that have been used widely as coolants and lubricants in transformers, capacitors, and other electrical equipment. Products containing PCBs are old fluorescent lighting fixtures, electrical appliances containing PCB capacitors, old microscope oil, and hydraulic fluids. The manufacture of PCBs stopped in the United States in 1977 because of evidence that they accumulate in the environment and cause harmful effects. PCBs are known to have estrogenic properties in humans, but the estrogenic effects are weak. 7 Importantly, PCB congeners are classified as estrogenic, antiestrogenic, or neither based on data from numerous research studies.10 This may explain, to some degree, why results of studies evaluating the effect of PCBs on breast cancer have been conflicting.
Electromagnetic fields (EMF) are the result of living in modern times. Wherever electricity is used, a relative magnetic and electric field is produced. This includes the household where appliances, CD players, televisions and radios, and the general wiring in the house all produce EMF. Of public concern is the EMF produced by high-voltage transmission wires running through neighborhoods. Theoretically, EMF became a suspected carcinogen when animal experiments demonstrated EMF suppression of melatonin secretion.11 Melatonin suppresses the formation of mammary tumors in rats and has been shown, in vitro, to block estrogen-induced proliferation of human breast cancer cells; therefore, it was reasoned, that decreased melatonin production might lead to increased risk of breast cancer.12
Epidemiologic studies assessing the effect of EMF exposures in occupational settings and by histories of electric blanket use have been inconclusive.13-16 The 1999 NIEHS Report on power-line frequency electric and magnetic fields presented a summary of the literature on breast cancer associations and noted the lack of evidence of any relationship.17 The challenge of EMF research remains accurate assessment of total exposure, particularly because the general population is likely exposed, at low doses, to ubiquitous and multiple sources. Additionally, because of the highly technical and costly instrumentation required to obtain actual EMF measurements, no published epidemiologic study on breast cancer has yet directly determined total EMF exposure.
C. History and Description of NE/MA Projects
Each project, in responding to the Request for Applications (RFA CA/ES-93-024; Appendix B) fulfilled the following criteria: (1) women study participants resided in the high-risk states included in the Congressional mandate; (2) environmental substances of interest were geographic-related factors and detectable by advanced quantitative methods or existing markers of exposure; (3) participant data on known breast cancer risk factors would be included in the final analyses; (4) research methods would include a sound epidemiologic study design; and (5) an interdisciplinary investigative team would be involved in the study.
Because projects were submitted as independent proposals prior to scientific review and funding, there were slight variations in research methods reflecting the areas of expertise of the investigators, accessible resources, available research facilities, and location of research site. Five of the six studies focused on the role of organochlorine pesticides and PCBs, while theremaining project proposed to assess the association between residential EMF radiation exposure and breast cancer risk.
Table 2 lists the principal investigators, institutional affiliations, and major laboratory and epidemiologic analyses comprising NE/MA. A summary table of funds received by each institution is included in Appendix C. Study outlines of each project, as described in the 1994 NCI press release(Appendix D), are presented in the following text.
| TABLE 2. The Northeast and Mid-Atlantic Breast Cancer Study | ||
|---|---|---|
| Investigator | Institution | Analyses |
| Jo Freudenheim, Ph.D. | SUNY* Buffalo | Organochlorines, carcinogen- metabolizing enzymes |
| Kathy Helzlsouer, M.D. | Johns Hopkins University | Nutrients, organochlorines, glutathione S-transferase |
| David Hunter, M.D., Sc.D. | Brigham and Women's Hospital, Harvard School of Public Health | Organochlorines, vitamin D, electric blankets |
| Cristina Leske, M.D. | SUNY* Stony Brook | Residential EMF exposure |
| Mary Wolff, Ph.D. | Mount Sinai School of Medicine | Organochlorines, carcinogen-metabolizing enzymes |
| Tongzhang Zheng, M.D., Sc.D. | Yale School of Medicine | Organochlorines in breast adipose tissue and serum |
| * State University of New York | ||
1. Environmental and Genetic Determinants of Breast Cancer
Jo Freudenheim, Ph.D., SUNY Buffalo
This study used a subset of 345 postmenopausal women participants, residents of Erie and Niagara counties of western New York, who had participated in a previous study on breast cancer. The main objectives were to examine the relationship of several organochlorines (PCB congeners, DDT, hexachlorobenzene [HCB], and mirex) and risk of postmenopausal breast cancer. Blood specimens previously collected (i.e., prior to diagnosis of breast cancer in study participants) were assayed for organochlorines, indicating past exposure to these compounds.
Because there are a variety of PCBs with different biological effects, individual PCB congeners were analyzed separately. Another research aim was to study the variability of genes that encode enzymes involved in the metabolism of carcinogens and other compounds. This led to the examination of whether interactions among PCB and DDT exposures, genes, and known or suspected breast cancer risk factors influence the occurrence of breast cancer.
2. Environmental Factors and Breast Cancer Risk in Maryland
Kathy Helzlsouer, M.D., Johns Hopkins University
Stored serum obtained in 1974 and 1989 from healthy women residing in Maryland were available as a resource for participants who developed breast cancer 20 years later (cases). For comparison, women from the same group who did not develop breast cancer were designated as controls, totaling a study population of 682 women. Laboratory measurements determined blood levels of organochlorines as well as antioxidant nutrients (antioxidants are thought to be protective against cancer). Researchers also characterized a specific gene, glutathione S-transferase, involved with the metabolism of environmental chemicals.
3. Environmental Risk Factors and Breast Cancer in the Nurses' Health Study
David Hunter, M.D., Sc.D., Brigham & Women's Hospital, Harvard
Data and stored blood serum samples were utilized from the Nurses' Health Study (NHS), an ongoing prospective cohort study established in 1976 with 121,700 women. Cohort members initially resided in 10 states representing the Northeast (Connecticut, Delaware, Maryland, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, and Vermont), the District of Columbia, the Midwest (Ohio, Michigan), the West (California), and the South (Florida, Texas). From the Northeast sector and the District of Columbia, 370 women who subsequently developed breast cancer (cases) and an equal number of women who did not develop breast cancer (controls) were enrolled in this study. Serum samples from all cases and controls were assessed for levels of vitamin D, PCBs, and DDE with the thought being that among cases, DDE and PCB levels should be higher and vitamin D levels should be lower than among the controls. In addition, data on electric blanket use (representing EMF exposure) collected in 1992 from NHS participants were analyzed to determine an association with breast cancer. Variation of breast cancer incidence was compared among the four designated regions, asking the question of whether known risk factors could account for the regional differences.
4. EMF and Breast Cancer on Long Island Study
M. Cristina Leske, M.D., SUNY Stony Brook
This population-based, case-control study examined the role of EMF on the risk of breast cancer in long-term residents of Long Island. Study participants included 1,205 women, 604 women with breast cancer and 601 women without breast cancer, who also are participating in the ongoing Long Island Breast Cancer Study Project (LIBCSP). Exposure to EMF was assessed in home interviews obtaining information on occupational and residential exposures, by spot and 24-hour magnetic field recordings, ground current measurements, wire coding, and personal monitoring for 24-hour measurements.
Although the SUNY Stony Brook and the LIBCSP studies involve Long Island women, the environmental exposures under study, research approaches, and investigative teams differ. After NE/MA had been launched, including this EMF project, Congress requested (Public Law 103-43 in June 1993) the LIBCSP studies, independent and unassociated with NE/MA, to investigate environmental causes of breast cancer in Nassau, Suffolk, and Schoharie Counties, New York, and in Tolland County, Connecticut. This research activity is ongoing and currently beginning data analyses.
5. Environmental and Genetic Determinants of Breast Cancer
Mary S. Wolff, Ph.D., Mount Sinai School of Medicine
A hospital-based, case-control study was conducted to investigate environmental factors related to breast cancer in the New York metropolitan area (New York City, Long Island, and New Jersey). An ethnically diverse group of 164 women with breast cancer and 342 women without breast cancer agreed to participate in the study. A major research objective addressed the role of organochlorines, specifically DDE, PCBs, and trans -nonachlor, a chlordane residue, as these chemicals are known to have estrogenic effects and are carcinogenic in laboratory animals. Another aspect of the study evaluated how environmental factors may be associated with breast cancer-related genes and certain carcinogen metabolizing genes.
6. Organochlorine Compounds and Risk of Breast Cancer
Tongzhang Zheng, M.D., Sc.D., Yale School of Medicine
The role of organochlorines and other environmental factors in breast cancer development was assessed in two regions of Connecticut. A total of 475 women with breast cancer and 502 women with benign breast disease (controls) were recruited from the Yale-New Haven and Tolland County hospitals. Levels of PCBs and organochlorine pesticides such as DDT, DDE, trans -nonachlor, oxychlordane, beta-benzene hexachloride, and hexachlorobenzene were assessed in blood samples as well as breast adipose tissue.
D. Collaborations
NIH program staff and the six investigative teams met immediately after the grant awards had been issued. Interactive discussions involved a comparison of similarities and differences among the funded studies, determination of whether standardization of epidemiologic and laboratory methodologies was feasible and desirable, and agreement that a flexible type of consortium network (U01 or cooperative agreement) would encourage and facilitate collaborations and communications among the six groups. Committees (Chemical, Questionnaire, Genetic Markers, and Data Analyses) were formed to address methodological issues shared by the six projects and to provide guidance, as needed, to NE/MA as the research proceeded.
Because draft questionnaires had been written prior to funding of projects, investigators discussed question categories for obtaining data specific for NE/MA and agreed to include them, if not already present, in the finalized questionnaires. These categories were to ask about EMF and radiation exposures (e.g., related to medical treatment), foods with specific nutrients, such as phytoestrogens, and environmental and occupational exposures that included pesticides. Representative NE/MA questions are presented in Appendix E. All questionnaires also obtained information on known and suspected breast cancer risk factors, medical and family histories, residential and occupational histories, and lifestyle and behavioral factors.
In addition, before conducting laboratory analyses, each investigative team arranged to incorporate quality control and assurance measures and to perform interlaboratory validation of assays for organochlorines and genetic markers. Levels of organochlorine compounds were determined with and without lipid adjustment.
The investigators and NIH staff continued to meet annually during the NE/MA funding period. As the five projects assessing organochlorines and breast cancer risk were completing their statistical analyses, it was agreed to subsequently combine data and to compare results across the projects. These comparative analyses have provided the opportunity to access data for approximately 1,400 breast cancer cases and 1,600 controls. A paper is in preparation describing the collective data and results, and it will be submitted to a scientific journal for publication.
PART II. RESULTS
A. Abstracts
The following abstracts were submitted for this Report by the six investigative teams, summarizing significant results from each project. A list of current NE/MA publications is included in Appendix F with key publications indicated by asterisks. Appendix G contains copies of the papers.
U01 CA/ES62995
Environmental and Genetic Determinants of Breast Cancer
Jo L. Freudenheim, Ph.D.
Department of Social and Preventive Medicine
State University of New York at Buffalo
Co-investigators: Christine B. Ambrosone, Peter Shields, John Vena, James R. Marshall, Saxon Graham, Kirsten Moysich, Paul Kostyniak
Major Findings
We found no evidence of an adverse effect associated with DDT, hexachlorobenzene (HCB), or mirex as determined by serum measurements. For PCBs, we found no increase in risk associated with total PCBs or with the total number of different congeners detected. We did find a modest (approximately 1.5-fold) increase in risk for women exposed to one group of PCBs, those with fewer chlorines. Because breast milk is one of the few ways that organochlorines can leave the body, we were interested in differences in risk for women who had and had not breastfed. We found that among women who had one or more children but who had not breastfed any of their children, there was a two- to three-fold increase in risk associated with increased exposure to some of the organochlorines. No such association was observed among women who had breastfed at least one child.
Regarding the genetic factors, we found that one factor, N-acetyltransferase 2 (NAT2), may be of importance for women who smoke. NAT2 is involved in the metabolism of possibly carcinogenic compounds found in cigarette smoke. There is variation in the rate of this metabolism because of a commonly occurring genetic variant. Among postmenopausal women, we found that among those with the slower variant, there was a dose-dependent increase in risk with smoking, both current smoking and past smoking. There was no effect of smoking on risk for those with the faster variant. Also, among premenopausal women, there was no effect of either NAT2 or smoking on risk. Of course, from a public health standpoint, there are numerous reasons to discourage smoking in the population; these findings point to yet another possible risk from smoking.
Accumulating epidemiologic evidence supports the belief that alcohol consumption may increase the risk of breast cancer. We studied genetic variation in the metabolism of alcohol by alcohol dehydrogenase 3 (ADH3), a rate-limiting enzyme in the metabolism of alcohol. We found that among premenopausal women, the variation in this enzyme affected the association of alcohol consumption with risk. Women with the higher risk variant who drank more experienced a 3.5-fold greater risk as compared with lighter drinkers with the high-risk variant and compared with both light and heavy drinkers with the lower risk variant. Among premenopausal women, there may be a group that is more genetically susceptible to an effect of alcohol on risk.
Significance
These findings indicate, in general, that there does not seem to be a strong risk associated with intake of organochlorines, although there may be some compounds that increase risk and there may be some subgroups who are more susceptible. The examination of risk in relation to interactions of genetic and environmental factors is a relatively new field. As with all epidemiologic studies, our findings regarding genetic variation and alcohol and smoking need to be examined in other populations. Further, there is a rapid expansion in information regarding other genetic factors that may be important to examine and may provide insight into groups who are more sensitive to particular exposures, like alcohol and smoking.
U01 CA/ES62988
Environmental Factors and Breast Cancer Risk in Maryland
Kathy J. Helzlsouer, M.D., M.H.S.
Department of Epidemiology
Johns Hopkins School of Hygiene and Public Health
Baltimore, Maryland
Co-investigators: Anthony J. Alberg, George W. Comstock, Sandra Hoffman, Paul Strickland
Major Findings
The risk of developing breast cancer among women with the highest concentrations of DDE was roughly one-half that among women with the lowest concentrations, whether based on 1974 concentrations (Ptrend = 0.02) or 1989 concentrations (Ptrend = 0.08). Women with the highest concentrations of total PCBs also were at lower risk of breast cancer, but the trends by concentration level were not statistically significant. Adjustment for known risk factors did not alter the associations. The strongest inverse association was observed among women diagnosed 16 to 20 years after blood donation. The results from this prospective, community-based nested case control study are reassuring. Even after long-term followup (up to 20 years), exposure to relatively high concentrations of DDE or PCBs was not associated with an excess risk of breast cancer (Helzlsouer et al., 1999). Associations between DDE and PCBs and breast cancer were similar when stratified by genotype for the following genes: catechol-O-methyltransferase (COMT), cytochrome P450c17 (CYP17), and glutathione S-transferases (GSTM1, GSTT1, and GSTP1).
No association was observed between folate, vitamin B-6, and homocysteine concentrations and breast cancer risk. Serum vitamin B-12, however, appeared to decrease risk in postmenopausal women. This is the first study to examine the vitamin B-12 relationship and to suggest a potentially modifiable risk factor that may be suitable as a preventive intervention (Wu et al., 1999).
Ascorbic acid concentrations were measured for cases and controls who participated in CLUE II (1989 cohort) for whom an aliquot of plasma had been stored with metaphosphoric acid to preserve vitamin C levels. No association was observed between ascorbic acid concentration and breast cancer development (Wu et al., manuscript submitted for publication).
Postmenopausal women homozygous for the low activity COMT allele were at increased risk for the development of breast cancer. This risk was strongest among women with a body mass index (BMI, weight/height2) greater than the median and among women who had the null genotype for GSTM1 (Lavigne et al., 1997).
The risk of breast cancer increases as the number of putative high-risk genotypes increased (Ptrend < 0.001). The results suggested that genetic variability in the glutathione S-transferase (GST) gene family may be associated with an increased susceptibility to breast cancer.
No association was observed between CYP17 and breast cancer risk.
Significance
From the viewpoint of etiology, these findings provide reassurance that environmental organochlorine compound exposure does not contribute to the development of breast cancer. Other environmental factors are likely to be relevant, given the findings of an association between GST genotypes and breast cancer risk. From the viewpoint of prevention, the potential modifying effects of micronutrients such as vitamin B-12 deserve particular attention, because these are factors that are amenable to practical preventive measures.
U01 CA/ES62984
Environmental Risk Factors and Breast Cancer in the Nurses' Health Study
David J. Hunter, M.B.B.S., Sc.D.
Brigham and Women's Hospital
Harvard School of Public Health
Boston, Massachusetts
Co-investigators: Susan E. Hankinson, Francine Laden, Mary S. Wolff, Lucas M. Neas, Graham A. Colditz, Frank E. Speizer, Paige E. Tolbert, Walter C. Willett
Major Findings
Our data do not support the hypothesis that exposure to DDT and PCBs increases the risk of breast cancer. Data on DDE were available on 236 breast cancer case-control pairs identified through June 1, 1992, and data on PCBs were available for 230 pairs. The median level of DDE was lower among cases than among controls (4.71 vs. 5.35 ppb, p = 0.14), as was the median level of PCBs (4.49 vs. 4.68 ppb, p = 0.72). The multivariate relative risk (RR) of breast cancer for women in the highest quintiles of exposure as compared with women in the lowest quintile was 0.72 for DDE, 95 percent confidence interval (CI) = 0.32–1.40, and 0.66 for PCBs, 95 percent CI = 0.32–1.37 (Hunter et al., 1997).
We continued followup through June 1, 1994, adding 136 postmenopausal invasive breast cancer cases and their matched controls to the study population. With the larger sample size, we evaluated the association of individual PCB congeners 118, 138, 153, and 180 with breast cancer risk and stratified on risk factors, including history of lactation, body mass index (BMI or weight/height2) and CYP1A1 polymorphisms. Again, there was no evidence of an association of breast cancer risk with plasma levels of DDE, total PCBs, or the individual PCB congeners (manuscript prepared).
We evaluated predictors of plasma concentrations of DDE and PCBs in the controls described by Hunter et al., 1997. We considered personal attributes such as age, serum cholesterol, region of residence, adiposity, lactation, and dietary intake. Age and cholesterol were significant positive predictors of both DDE and PCBs. Women living in the western United States had higher levels of DDE (mean = 11.0 ppb; p = 0.003), and women in the Northeast and Midwest had higher levels of PCBs (mean = 5.6 ppb; p = 0.0002) as compared with women from other parts of the country (mean DDE = 6.3; mean PCBs = 4.5 ppb). Levels of DDE could not be predicted from consumption of different food groups. There was a positive association between fish consumption and PCB concentrations among women in the Northeast and Midwest. The null results for the majority of food variables suggest that specific dietary factors, other than fish, are not currently a substantial contributor to human exposure to DDE and PCBs (Laden et al., 1999).
We identified 2,603 incident cases of invasive breast cancer through 1992 (1,794,565 person-years of followup). We calculated RRs comparing California, the Northeast, and the Midwest with the South. For premenopausal women, there was little evidence of regional variation in breast cancer incidence rates. For postmenopausal women in California, age-adjusted risk was modestly elevated (RR = 1.24; 95% CI = 1.05–1.47). After adjusting for established risk factors, the excess rate in California was attenuated by 25 percent (RR = 1.18; 95% CI = 1.00–1.40). No excess of breast cancer incidence was observed for postmenopausal women in either the Northeast or the Midwest. In conclusion, we observed little regional variation in age-adjusted breast cancer incidence, with the exception of a modest excess for postmenopausal women in California. Adjustment for differences in the distribution of established risk factors explained some of the excess risk in California (Laden et al., 1997).
On the biennial questionnaire in 1992, 87,497 women provided information on this exposure during three consecutive time periods. In a prospective analysis with 301,775 person-years of followup through 1996 (954 cases), the RR for any electric blanket use was not elevated (RR = 1.08; 95% CI = 0.95–1.24) after controlling for breast cancer risk factors. There was a weak association between breast cancer and electric blanket use at least 16 years before diagnosis and for long-term use in age-adjusted analyses, but not in multivariate models. In a retrospective analysis of 1,318,683 person-years of followup (2,426 cases), the multivariate RR associated with use before disease was null (RR = 1.05; 95% CI = 0.95–1.16). Although 95 percent confidence intervals for these estimates did not exclude small risks; overall, results did not support an association between breast cancer risk and exposure to EMF from electric blankets (in press).
U01 CA/ES62991
EMF and Breast Cancer on Long Island Study
M. Cristina Leske, M.D., M.P.H.
Department of Preventive Medicine, Division of Epidemiology
University Medical Center at Stony Brook, New York
Co-investigators: Elinor R. Schoenfeld, Roger Grimson, Geoffrey Kabat
Due to the technical requirements of EMF measurements, we began with a comprehensive preliminary survey, a pilot study, to determine where home exposures should be obtained (Schoenfeld et al., 1999). Residential measurements that best predicted personal exposures were mean 24-hour measurements in the bedroom and in the most frequently lived-in room, as well as ground current test load measurements taken at the center of this most lived-in room. Based on these findings, the study protocol included a 1-hour home visit to conduct a personal interview and to perform in-home EMF measurements. The interview asked participants for histories of occupational and residential EMF exposures, including appliance use. Power lines adjacent to the home also were diagrammed (wire coded). Thus, the study addressed the issue of exposure assessment by incorporating several methods, including ground current measurements, which have not been used before in the context of an epidemiologic study.
Total EMF exposure measurements and questionnaire data on known and suspected breast cancer risk factors have been entered into databases, and data analyses are ongoing.
U01 CA/ES62951
Environmental and Genetic Determinants of Breast Cancer
Mary S. Wolff, Ph.D.
Department of Community Medicine
Mount Sinai School of Medicine
New York, New York
Co-investigators: Gertrud Berkowitz, Ira Bleiweiss, Steven Brower, Geoffrey Kabat, Benjamin Pace, Ruby Senie, Paul Tartter, Sylvan Wallenstein, Ainsley Weston
Major Findings
- Organochlorides (DDT, PCBs, trans -nonachlor) have been determined in serum. Results show higher levels of DDE among the minority women (11 ppb on average vs. 6 ppb among whites) and higher levels of PCBs and trans -nonachlor among African-Americans.
- An assay for the phytoestrogens (e.g., daidzein and genistein) and other antioxidants in urine has been established, and preliminary data show detectable levels in 20 to 50 percent of women. These biological markers of exposure are a useful corollary to the dietary histories, which are complete on all women and are being analyzed.
- Hispanic and black women have a higher frequency (40 percent) of the minor rapid-metabolizer allele in CYP1A1 than do non-Hispanic white women (< 20 percent).
- In the case of NAT2, Hispanic and white women have higher frequency of the null NAT2 genotype (60 to70 percent) compared with black women (< 40 percent).
- The distribution of GST (theta and mu genotypes) nonfunctional alleles was similar among the three ethnic groups.
- The minor CYP2E1 variant was slightly more common in minority women.
- We also have investigated the haplotype of three biallelic polymorphisms in P53 and BRCA1, using a new method developed in our laboratory. Among 290 women, six of eight possible P53 haplotypes so far have been detected among 580 chromosomes. One specific haplotype may be overrepresented in Caucasian breast cancer cases (Weston et al., 1997). Genotypes now have been completed for 530 women, and the data are being analyzed.
- For BRCA1, we have reported in an abstract that one haplotype consisting of six biallelic polymorphisms is found almost exclusively in Caucasians, and this haplotype was associated with a RR of 2.0 in heterozygotes and greater than 8.0 in homozygotes (Weston et al., 1997).
- We have described frequencies of a polymorphism in CYP17. Results did not confirm an earlier report showing an association with age at menarche and with tumor aggressivity (Weston et al., 1998). We also have completed caffeine phenotyping of 177 women for CYP1A2.
Significance
Minority women in New York City have higher levels of organochlorines and different distributions of several metabolizing genes and breast cancer susceptibility genes. The association between specific environmental factors and breast cancer risk is being analyzed presently.
U01 CA/ES62986
Organochlorine Compounds and Risk of Breast Cancer
Tongzhang Zheng, M.D., Sc.D.
Department of Epidemiology and Public Health
Yale School of Medicine
New Haven, Connecticut
Co-investigators: Theodore Holford, John Tessari, Susan T. Mayne, Barbara Ward, Darryl Carter, Patricia H. Owens, Peter Boyle, Sheilia H. Zahm
Major Findings
We found no significantly increased risk of breast cancer associated with breast adipose tissue levels of PCBs or organochlorine pesticides, including DDT, DDE, trans -nonachlor, oxychlordane, β-benzene hexachloride, and hexachlorobenzene. Further stratification by menopausal status, parity, lactation status, estrogen receptor and progesterone receptor status also showed no significant associations with breast adipose levels of PCBs or organochlorine pesticides. An assessment of the joint effects for DDE and PCBs did not show a significant interaction between the two factors on the risk of female breast cancer. We did not see a significant association between breast cancer risk and serum levels of PCBs or organochlorine pesticides.
Significance
We conclude that our study does not support the hypothesis that organochlorine pesticides and PCBs, as encountered through environmental exposure, increase the risk of female breast cancer.
B. Summary
Although research questions and types of data collected in NE/MA were similar among projects, some methodological differences existed for reasons cited in Part I (Background, section C). Variation also exists in participant recruitment (for example, prior to or after the diagnosis of breast cancer for cases), demographics, the timing of biological specimen collection, and which specific components, whether related to environmental factors or to genetic makeup of individuals, were analyzed in the laboratories. These differences are accounted for in the data interpretation and conclusions of each project; however, merging of data across projects for one major analysis or meta-analysis was not possible.
The variation is a strength of NE/MA, which led to the discovery of new findings unique to the characteristics of the reporting project. It contributed to the enhancement of research models that will be useful for other investigators planning environmental studies of breast cancer. For example, there was refinement in analytical approaches for congener-specific PCBs18 and in validation of blood and breast adipose tissue levels of organochlorine pesticides.19
The NE/MA investigators used the case-control study design, either within prospective cohorts or as hospital-based, to determine whether exposure to organochlorines and electromagnetic fields could explain a geographic-related observation of breast cancer rates. Three projects included an exploration of any possible evidence of gene-environment interactions and breast cancer occurrence. Also of interest was the influence of protective factors such as specific dietary nutrients (as antioxidants) and history of breastfeeding. Consolidation of reported findings follows.
Organochlorines
DDT and DDE. There does not appear to be an increased risk of breast cancer from DDT exposure or the presence of DDT metabolite (formed in the body), DDE. Exposure levels, even when considered in the high range, did not appear to confer this risk. One investigative team reported that higher levels of DDE were found in minority women (African- and Hispanic-Americans) as compared with Caucasians in their study; however, whether this is associated with a higher risk of breast cancer remains to be determined.
PCBs. Measurements of total PCBs did not increase the risk of breast cancer. One project, in analyzing a subgroup of women, found a small significant relationship between PCBs and breast cancer in postmenopausal women who had not breastfed an infant. The investigators also reported cancer risk in women exposed to the less chlorinated PCB congeners.
Electromagnetic Fields
Preliminary results suggest, in two projects, that long-term exposure to EMF represented by electric blanket use does not, in general, increase the risk of breast cancer. Further analyses are ongoing.
Genetic Factors
Analyses of variations (polymorphisms) in certain genes, those involved in the process of carcinogen metabolism, presented promising clues for additional investigations. The slower variant of NAT2 increased breast cancer risk in postmenopausal women who are smokers. The group of genes known as GST was found to increase risk, but only in relation to the number of high-risk genotypes (GSTM1, GSTP1, and GSTT1) present. Elevated breast cancer risk was reported for a subpopulation of postmenopausal women, particularly with higher than average BMI (weight/height2), who also carried a certain allele of the COMT gene.
Dietary and Behavioral Factors
Analyses of dietary components have been reported in detail by two projects. No difference was observed between breast cancer cases and controls when intake of folate, homocysteine, or vitamins B-6 and C were analyzed singly or in the presence of organochlorines; however, serum vitamin B-12 may be protective. Genetic variation in an enzyme involved in metabolism of alcohol, alcohol dehydrogenase 3 (ADH3), was found to influence the effect of alcohol consumption on a premenopausal woman's susceptibility to develop breast cancer.
PART III. CONCLUSIONS
Although strides have been made toward understanding breast cancer as a disease, particularly in molecular biology and genetics, a desired goal is to alter its incidence and mortality. Screening and prevention programs nationwide are committed to this goal and already have begun to make an impact. Breast cancer death rates in the United States have been decreasing about 2 percent per year since 199020 (Table 1), although occurrence has not changed substantially. Identification of modifiable risk factors offers the greatest opportunity to change incidence rates. The question of whether any environmental factor (e.g., substances present outside of the body, including diet-related components and alcohol) is associated with risk has been pursued by many investigators with the hope of developing prevention strategies. Due to the complexity of the question and of breast cancer itself, it is not expected that any one group of studies, research approach, or scientific discipline will be able singly to provide an answer.
What is to be learned from NE/MA?
- In the northeastern and mid-Atlantic regions of the United States, no evidence was found that organochlorines play a role in breast cancer development.
As NE/MA was being conducted, other epidemiologic studies21,22 reported similar findings in U.S. study populations. Ongoing studies in areas with high breast cancer rates will be of particular interest as data are analyzed in the year 2000: (1) the Long Island Breast Cancer Study; (2) a hospital-based study in Long Island; and (3) a study of pregnant women from a California cohort established in the 1960s who subsequently developed breast cancer.
Because DDT use and manufacturing of PCBs have ceased in the United States for more than 20 years, blood levels have been decreasing (DDT more so than PCBs, which are still in use in transformers), reflecting the lack of exposure to these compounds. Studies conducted in countries where DDT has not yet been banned such as Mexico, Colombia, Brazil, and Vietnam have observed no breast cancer association except for the Colombian women who were exposed to the highest DDT concentrations.23-26 Three European studies have reported conflicting results.27-29 Although DDT was not implicated in any study, the pesticides dieldrin and beta-hexachlorocyclo-hexane (beta-HCH) were linked to breast cancer risk in a Danish study population,27 while German investigators reported a positive relationship between certain PCB congeners and breast cancer.28
- NE/MA results suggest that, within the population exposed to environmental substances, there may be subgroups of women who are at higher breast cancer risk.
Increased breast cancer susceptibility could be related to differences in an individual's capacity to metabolize (break down or transform chemically) chemical substances, a process associated with certain genes. There also may be characteristics (e.g., menopausal status, body mass index or BMI) and behavioral patterns (e.g., breastfeeding, tobacco consumption) that interact with the genes to increase or decrease risk. This exciting new area of scientific research, called gene-environment interaction, combines laboratory advances in molecular genetics with epidemiology. The NCI and NIEHS have designated this research area as a high priority (i.e., an "extraordinary opportunity for investment") in the NCI Bypass budget and the NIEHS Environmental Genome Project.
The study of gene-environment interactions requires a multidisciplinary approach. The intensive search for breast cancer-related genes is a major research effort of NCI investigators and funded scientists in the community. Parallel to this activity are studies that are trying to delineate the effect of potentially modifiable factors such as diet and nutrition, exercise, and hormones (NCI Breast Cancer Progress Review Group Report, 1998). The search for relevant environmental factors is the basis for several current initiatives co-sponsored by NIH institutes, the Centers for Disease Control and Prevention (CDC), and the Environmental Protection Agency (EPA).
Future Research Directions: Breast Cancer and the Environment
- Research Approach
Human population studies that plan to assess multiple factors together, such as cancer genes, environmental exposures, lifestyle and behavioral characteristics, require large numbers of study participants and biological specimens for quantitating exposures. This endeavor may necessitate investigator networks and new strategies to collaborate in jointly ascertaining study populations and establishing shared infrastructures and specimen repositories. The ideal research approach is the prospective or cohort study design where healthy individuals are followed with collection of appropriate environmental, epidemiologic, and clinical data until breast cancer is diagnosed. This would, however, be a long-term, costly commitment involving thousands of study participants, and research sites should have high exposure levels of suspected carcinogens. An alternative possibility may be the use of previously assembled cohorts with stored specimens that served other research purposes; however, critical exposure data may not be available. This emphasizes the need for better research methods and instrumentation to determine retrospective environmental exposures.
- Susceptibility and Gene-Environment Interactions
Much interest has been generated regarding genetic determinants (genotypes) of enzymes that metabolize environmental chemicals or endogenous hormones in the body. Increased risk of breast cancer associated with specific genetic polymorphisms, as suggested by NE/MA results, require confirmation in subpopulations, and studies of ethnically diverse groups with differing breast cancer rates may be especially enlightening. Continuing research is needed to delineate the basis of an individual's susceptibility to breast cancer and to determine what may be the contribution of environmental factors. Collaborations will require integration of research effort by epidemiologists, geneticists, molecular and cell biologists, endocrinologists, environmental scientists, and biostatisticians.
- Time Period of Exposure
Laboratory research has demonstrated that breast tissue in certain time periods of development, particularly related to endogenous hormones (mainly estrogens), is susceptible to the effect of carcinogens. In a woman's life, it is hypothesized that the time of susceptibility is between age at menarche and age at first full-term pregnancy. It also has been suggested that because there are high levels of hormones, notably estrogens during pregnancy, in utero exposure may contribute to adult breast cancer in susceptible female offspring.30 Attentiveness to time of environmental exposures (e.g., during prenatal, adolescent, reproductive, and postmenopausal periods when hormonal activity may increase breast tissue susceptibility) could lead to new insight into the pathogenesis of breast cancer.
- Exposure Assessment
Much remains unknown regarding environmental links to the origin of breast cancer, although this is an active area of research. Identification and evaluation of potential hazardous agents and chemicals presently are included in activities of the NIEHS, Environmental Protection Agency (EPA), Food and Drug Administration (FDA), Agency for Toxic Substances and Disease Registry (ATSDR), National Toxicology Program (NTP), and National Institute of Occupational Safety and Health (NIOSH) in the Centers for Disease Control and Prevention (CDC). Future basic research characterizing biological effects of environmental chemicals, particularly those that may have estrogenic activity such as endocrine disruptors, and developing models to determine what influences body burden of suspected carcinogens (e.g., persistent pesticides in the food chain) will facilitate setting priorities for conducting epidemiologic studies.
Although laboratory testing of chemical carcinogens of mammary glands in rodents is well-established and toxicological effects can be determined, the doses used in animals cannot be correlated directly to exposures encountered by the general population. Moreover, substances in the ambient environment generally come from multiple sources, are present at low or undetectable concentrations or as complex mixtures, and vacillate in time and place. Thus, a major challenge for breast cancer epidemiologists is to develop methods, in collaboration with environmental scientists, for accurate determination of environmental exposures, spanning 20-30 years of an individual's life, relevant to breast cancer development.
This report was submitted by NIH staff in December 1999, and prepared for publication by the Scientific Consulting Group, Inc. (SCG), Gaithersburg, Maryland, under the direction of Darrell E. Anderson.
REFERENCES
- Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin 1999;49:8-31.
- National Cancer Institute. Cancer Research: Because Lives Depend on It. July 1999.
- Devesa SS, Grauman DJ, Blot WJ, Pennello, GA, Hoover RN, Fraumeni JF Jr. Atlas of Cancer Mortality in the United States, 1950-94. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, NIH publication no. 99-4564; 1999:18.
- Sturgeon SR, Schairer C, Gail M, McAdams M, Brinton LA, Hoover RN. Geographic variation in mortality from breast cancer among white women in the United States. J Natl Cancer Inst 1995;87:1846-1853.
- Falck F Jr, Ricci A Jr, Wolff MS, Godbold J, Deckers P. Pesticides and polychlorinated biphenyl residues in human breast lipids and their relation to breast cancer. Arch Environ Health 1992;47:143-146.
- Wolff MS, Toniolo PG, Lee EW, Rivera M, Dubin N. Blood levels of organochlorine residues and risk of breast cancer. J Natl Cancer Inst 1993;85:648-652.
- Safe SH and Zacharewski T. Organochlorine exposure and risk for breast cancer. In: Etiology of Breast and Gynecological Cancers, Aldaz, Gould, McLachan, and Slaga, eds. New York: Wiley-Liss, 1997;133-145.
- Moysich KB, Ambrosone CB, Vena JE, Shields PG, Mendola P, Kostyniak P, Greizerstein H. H, Graham S, Marshall JR, Schisterman EF, Freudenheim JL. Environmental organochlorine exposure and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 1998;7:181-188.
- IARC Working Group.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (Vol.56), Lyon, International Agency for Research on Cancer, 1993.
- Wolff MS and Toniolo P. Environmental organochlorine exposure as a potential etiologic actor in breast cancer. Environ Health Persp 1995;103 (Suppl 7):141-145.
- Stevens RG, Davis S, Thomas D, Anderson LE, Wilson BW. Electric power, pineal function and the risk of breast cancer. FASEB J 1992;6:853-860.
- Brainard GC, Kavet R, Kheifets LI. The relationship between electromagnetic field and light exposures to melatonin and breast cancer risk: a review of the relevant literature. J Pineal Res 1999;26:65-100.
- Gammon MD, Schoenberg JB, Britton JA, Kelsey JL, Stanford JL, Malone KE, Coates RJ, Brogan DJ, Potischman N, Swanson CA, Brinton LA. Electric blanket use and breast cancer risk among younger women. Am J Epidemiol 1998;148:556-563.
- Vena JE, Graham S, Hellmann R, Swanson M, Brasure J. Use of electric blankets and risk of postmenopausal breast cancer. Am J Epidemiol 1991;134:180-185.
- Feychting M, Forssen U, Rutqvist LE, Ahlbom A. Magnetic fields and breast cancer in Swedish adults residing near high-voltage power lines. Epidemiology 1998;9:393-397.
- Coogan PF, Clapp RW, Newcomb PA, Wenzl TB, Bogdan G, Mittendorf R, Baron JA, Longnecker MP. Occupational exposure to 60-Hertz magnetic fields and risk of breast cancer in women. Epidemiology 1996;7:459-464.
- National Institute of Environmental Health Sciences. NIEHS Report on Health Effects From Exposure to Power-Line Frequency Electric and Magnetic Fields: Prepared in Response to the 1992 Energy Policy Act (PL 102-486, Section 2118). Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, NIH publication no. 99-4493; 1999.
- Moysich KB, Mendola P, Schisterman EF, Freudenheim JL, Ambrosone CB, Vena JE, Shields PG, Kostyniak P, Greizerstein H, Graham S, Marshall JR. An evaluation of proposed frameworks for grouping polychlorinated biphenyl (PCB) congener data into meaningful analytic units. Am J Ind Med 1999;35(3):223-231.
- Archibeque-Engle SL, Tessari JD, Winn DT, Keefe TJ, Nett TM, Zheng T. Comparison of organochlorine pesticide and polychlorinated biphenyl residues in human breast adipose tissue and serum. J Toxicol Environ Health 1997;52:285-293.
- Wingo PA, Ries LAG, Giovino, GA, Miller, DS, Rosenberg HM, Shopland DR, Thun MJ, Edwards BK. Annual report to the nation on the status of cancer, 1973-1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst 1999;91(8):675-690.
- Krieger N, Wolff MS, Hiatt RA, Rivera M, Vogelman J, Orentreich N. Breast cancer and serum organochlorines: a prospective study among white, black, and Asian women. J Natl Cancer Inst 1994;86:589-599.
- Dorgan JF, Brock JW, Rothman N, Needham LL, Miller R, Stephenson, Jr. HE, Schussler N, Taylor PR. Serum organochlorine pesticides and PCBs and breast cancer risk: results from a prospective analysis (USA). Cancer Causes Control 1999;10:1-11.
- Lopez-Carrillo L, Blair A, Lopez-Cervantes M, Cebrian M, Rueda C, Reyes R, Mohar A, Bravo J. Dichlorodiphenyltrichloroethane serum levels and breast cancer risk: a case-control study from Mexico. Cancer Res 1997;57:3728-3732.
- Olaya-Contreras P, Rodriguez-Villamil J, Posso-Valencia HJ, Cortez JE. Organochlorine exposure and breast cancer risk in Colombian women. Cad Saude Publica 1998;14(Suppl 3): 125-132.
- Mendonca GA, Eluf-Neto J, Andrada-Serpa MJ, Carmo PA, Barreto HH, Inomata ON, Kussumi TA. Organochlorines and breast cancer: a case-control study in Brazil. Int J Cancer 1999;83:596-600.
- Schecter A, Toniolo P, Dai LC, Thuy LT, Wolff MS. Blood levels of DDT and breast cancer risk among women living in the north of Vietnam. Arch Environ Contam Toxicol 1997;33:454-456.
- Hoyer AP, Grandjean P, Jorgensen T, Brock JW, Hartvig HB. Organochlorine exposure and risk of breast cancer. Lancet 1998;352:1816-1820.
- Guttes S, Failing K, Neumann K, Kleinstein J, Georgii S, Brunn H. Chlororganic pesticides and polychlorinated biphenyls in breast tissue of women with benign and malignant breast disease. Arch Environ Contam Toxicol 1998;35:140-147.
- van't Veer P, Lobbezoo IE, Martin-Moreno JM, Guallar E, Gomez-Aracena J, Kardinaal AF, Kohlmeier L, Martin BC, Strain JJ, Thamm M, van Zoonen P, Baumann BA, Huttunen JK, Kok FJ. DDT (dicophane) and postmenopausal breast cancer in Europe: case-control study. BMJ 1997;315:81-85.
- Ekbom A, Hsieh CC, Lipworth L, Adami HQ, Trichopoulos D. Intrauterine environment and breast cancer risk in women: a population-based study. J Natl Cancer Inst 1997;89(1):71-76.
The information on this page is archived and provided for reference purposes only.