EGRP Cancer Epidemiology News
December 2023
- 2023: Year in Review
- Additional Funding Opportunities
- Job and Training Opportunities
- Research Resources
- News and Blog Posts
2023: Year in Review

Message from Acting Associate Director, Epidemiology and Genomics Research Program
Greetings,
As the year comes to a close, we once again express our gratitude for the leadership of Dr. Kathy Helzlsouer, MD, MHS, who retired in October after a decades-long career of research and service.
In 2022, NCI’s Division of Cancer Control and Population Sciences (DCCPS) identified new areas of opportunity – health equity, data strategies, modifiable risk factors, digital health, evidence-based policy research, and climate change. The 2023 DCCPS Overview and Highlights report outlines progress made on these priorities by staff across the division, including many from the Epidemiology and Genomics Research Program (EGRP).
Along with reflecting on DCCPS’s progress, we have also been considering EGRP’s progress. In this newsletter, we highlight new additions to EGRP’s roster of program directors, new funding opportunities our staff have developed in the past year, and some of our virtual scientific workshops and webinars, many of which were recorded for those who were not able to participate in the live events. Additionally, we invite you to explore some of EGRP’s research resources that were updated this year.
On behalf of all my colleagues in EGRP, we wish you all peace, health, and happiness throughout the New Year.
Warmest regards,
Pothur Srinivas, PhD, MPH
New EGRP Program Directors

In November, Andrea Burnett-Hartman, PhD, MPH joined EGRP’s Clinical and Translational Epidemiology Branch (CTEB). Her responsibilities include managing a research portfolio of grants focused on clinical, lifestyle, and genomic factors that affect cancer outcomes, including those factors that may contribute to observed disparities in health outcomes.
Prior to joining NCI, Dr. Burnett-Hartman spent 15 years as an investigator in multi-site, collaborative research initiatives at the Fred Hutchinson Cancer Center and the Kaiser Permanente Colorado Institute for Health Research. These included the Population-based Research to Optimize the Screening Process Consortium, Cancer Research Network, National Cancer Institute Cohort Initiative, Kaiser Permanente Research Bank, Genetics and Epidemiology of Colorectal Cancer Consortium, and Patient Outcomes to Advance Learning Colorectal Cancer Network. She also served on the National Leadership Team for a multi-site biobank initiative and is experienced in building research cohorts to study cancer-related outcomes.

Also in November, Naoko Ishibe, ScD joined EGRP’s Environmental Epidemiology Branch (EEB). Her responsibilities include overseeing a portfolio of grants and initiatives that support research on modifiable risk factors and cancer etiology and outcomes, particularly as they relate to childhood and early-onset cancers.
Dr. Ishibe first joined EGRP as a program director in CTEB, where she oversaw and managed a research grant portfolio that included epidemiologic and molecular studies associated with cancer susceptibility and cancer-related outcomes. She also provided scientific leadership and direction for NCI workshops that resulted in publications – the Workshop on Comparative Effectiveness Research in Genomics in Personalized Medicine and the Survivorship Cohort Workshop. Thereafter, Dr. Ishibe was a consultant to EGRP, collaborating with government scientists to conduct and review the state of the science and actively identifying research gaps of high priority to EGRP across the cancer care continuum. Prior to joining EGRP, Dr. Ishibe was a study director at the National Academies of Sciences, Engineering, and Medicine.

In August, Behnoosh Momin, DrPH, MS, MPH joined EEB. Her responsibilities include managing a portfolio of research grants to promote and support epidemiologic research on modifiable risk factors and cancer in diverse populations to advance the prevention and control of cancer.
Prior to joining EGRP, Dr. Momin was a health scientist in the Centers for Disease Control and Prevention’s (CDC) Division of Cancer Prevention and Control’s Comprehensive Cancer Control Branch, Scientific Support and Clinical Translation Team. Her role involved providing scientific expertise to a diverse range of studies. Notably, she served as a project lead on an American Recovery and Reinvestment Act-funded research study aimed at identifying promotional and cessation interventions for tobacco control to reduce cancer rates. Dr. Momin also served as co-lead on a study measuring the effects of state and local radon policies. She is the co-founder of CDC’s tobacco related cancers workgroup, which promotes tobacco related cancer epidemiology research. Her most recent efforts have been dedicated to leading liver cancer research demonstration projects across US states, tribes, and territories funded under the National Comprehensive Cancer Control Program.
EGRP’s complete staff list can be viewed at https://epi.grants.cancer.gov/staff/.
Supporting Research
Cannabis Use During Cancer Treatment
This $15 million initiative, Cannabis and Cannabinoid Use in Adult Cancer Patients During Treatment: Assessing Benefits and Harms, funds five prospective cohorts of patients undergoing active cancer treatment across the country, longitudinally investigating the potential benefits and harms of cannabis use. NCI is co-funding the initiative with the National Center for Complementary and Integrative Health (NCCIH).
Smart Health and Biomedical Research
EGRP once again joined the Smart Health and Biomedical Research in the Era of Artificial Intelligence and Advanced Data Science funding opportunity reissued by the National Science Foundation. This initiative will support innovative, high-risk/high-reward research with the promise of disruptive transformations in biomedical and public health research. The solicitation aims to address technological and data science challenges that require fundamental research and development of new tools, workflows, and methods across many dimensions.
EGRP’s Grants Portfolio and Funding Opportunities in 2023
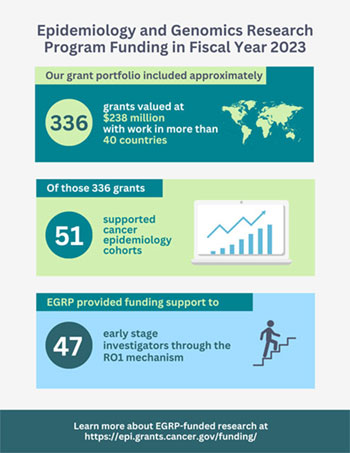
EGRP joins with other divisions, offices, and centers at NCI and other NIH institutes to fund investigator-initiated research and applications submitted in response to funding opportunities. EGRP supports projects in the United States and globally. We are happy to report that the overall number of funded projects that EGRP oversees increased in fiscal year 2023 to 336 grants valued at $238 million, including 47 early-stage investigators.
Learn more about active cancer epidemiology projects funded by EGRP.
EGRP staff served as scientific contacts for more than 25 new and re-issued funding opportunities in 2023.
Funding opportunities EGRP staff were involved with in 2023 that are still accepting applications include the following:
- Informatics Technologies for Cancer Research and Management
- RFA-CA-24-016, Development of Methods and Algorithms (R21, Clinical Trial Optional)
- RFA-CA-24-017, Early-Stage Development (U01, Clinical Trial Optional)
- RFA-CA-24-018, Advanced Development (U24, Clinical Trial Optional)
- RFA-CA-24-019, Sustained Support (U24, Clinical Trial Optional)
- RFA-HG-23-017, Investigator-Initiated Research in Genomics and Health Equity (R01, Clinical Trial Optional)
- Advancing Genomic Medicine Research
- RFA-HG-23-032 (R01, Clinical Trial Optional)
- RFA-HG-23-033 (R21, Clinical Trial Optional)
- Co-infection and Cancer
- PAR-23-055 (R01, Clinical Trial Not Allowed)
- PAR-23-056 (R21, Clinical Trial Not Allowed)
- PAR-23-058, NCI Small Grants Program for Cancer Research for Years 2023, 2024, and 2025 (R03, Clinical Trial Optional)
- PAR-23-075, Small Research Grants for Analyses of Gabriella Miller Kids First Pediatric Research Data (R03, Clinical Trial Not Allowed)
- PAR-23-124, Genomic Community Resources (U24, Clinical Trial Not Allowed)
- Impacts of Climate Change across the Cancer Control Continuum
- PAR-23-152 (R21, Clinical Trial Optional)
- PAR-23-153 (R01, Clinical Trial Optional)
- PAR-23-199, ClinGen Genomic Curation Expert Panels (U24, Clinical Trial Not Allowed)
- Secondary Analysis and Integration of Existing Data to Elucidate Cancer Risk and Related Outcomes
- PAR-23-254 (R01, Clinical Trial Not Allowed)
- PAR-23-255 (R21, Clinical Trial Not Allowed)
- Developing Novel Theory and Methods for Understanding the Genetic Architecture of Complex Human Traits
- PAR-23-301 (R21, Clinical Trial Not Allowed)
- PAR-23-302 (R01, Clinical Trial Not Allowed)
- Assay Validation of High Quality Markers for Clinical Studies in Cancer
- PAR-23-313 (UH2/UH3, Clinical Trial Not Allowed)
- PAR-23-314 (UH3, Clinical Trial Not Allowed)
- Ethical, Legal and Social Implications (ELSI) Research
- PAR-23-293 (UH2/UH3, Clinical Trial Not Allowed)
- PAR-23-294 (UH3, Clinical Trial Not Allowed)
- PAR-23-295 (UH3, Clinical Trial Not Allowed)
- NOT-CA-23-004, Notice of Special Interest (NOSI): Utilization of Cohorts and Prospective Study Designs for Liquid Biopsy Assay Validation for Early Detection of Cancers
- NOT-CA-23-037, NOSI: Technology Development for Cancer Control and Population Science Research
- NOT-CA-23-070, NOSI: Research on HIV-associated Malignancies
- NOT-CA-23-078, NOSI: Pragmatic Trials in Low-Resource Settings
- NOT-HL-23-001, NOSI: Epidemiologic Studies in Asian Americans, Native Hawaiians, and Pacific Islanders (Parent R01, Clinical Trial Not Allowed)
- NOT-MD-23-002, NOSI: Addressing the Etiology of Health Disparities and Health Advantages Among Immigrant Populations
Funding opportunities EGRP staff were involved with in 2023 that are no longer accepting applications include:
- Advancing Cancer Control Equity Research Through Transformative Solutions
- RFA-CA-23-026 (U19, Clinical Trial Optional)
- RFA-CA-23-027 (U24, Clinical Trial Optional)
- RFA-CA-23-023, Liver Cancer Collaborative Projects with the Liver Cirrhosis Network (U01, Clinical Trial Optional)
- Network of Genomics-Enabled Learning Health Systems (gLHS) – Clinical Sites
- RFA-HG-23-041 (U01, Clinical Trial Required)
- RFA-HG-23-042 (U01, Clinical Trial Required)
- NOT-CA-23-046, NOSI: Administrative Supplements to Conduct Systematic Evidence Reviews on the Clinical Utility of Cancer-site Specific Polygenic Risk Scores (PRS) for Cancer Risk Assessment
- NOT-CA-23-048, Enhancement of External Environmental Exposure Assessment for Cancer Epidemiology Research
- (Interagency program solicitation) Smart Health and Biomedical Research in the Era of Artificial Intelligence and Advanced Data Science
More information about funding opportunities sponsored or co-sponsored by DCCPS are available on the DCCPS funding opportunities web page. Visit EGRP’s funding and grants web page for links to additional NCI and NIH programs with FOAs relevant to cancer epidemiologists.
Scientific Meetings and Webinars
In collaboration with other NCI and NIH programs, EGRP staff planned several scientific meetings to bring together experts and interested individuals to collaborate regarding solutions to scientific questions and research needs.
- On February 7-8, 2023, EGRP hosted the Variation to Biology: Optimizing Functional Analysis of Cancer Risk Variants workshop. The event was designed to identify and discuss how best to address scientific challenges and opportunities for understanding the path from genetic variation to cancer phenotype. The agenda and recordings of the sessions are available on the workshop website.
- Planning for Integrating Environmental Data with Other Omics for Cancer Epidemiology, a joint NIEHS-NCI workshop, was led by EGRP staff, who also participated in the meeting. Held on February 14-15, 2023, the purpose of the workshop was to identify the challenges and opportunities related to the integration of environmental exposure data with other omics data for human cancer population studies and to inform future supported research directions at NIH. The workshop report is now available.
- EGRP staff were involved with NIH support efforts for the Active Living Conference
 , held on March 13-16, 2023. The conference assembled a diverse group of researchers, practitioners, and policy makers to advance knowledge and action on how to evaluate, create and sustain active living environments.
, held on March 13-16, 2023. The conference assembled a diverse group of researchers, practitioners, and policy makers to advance knowledge and action on how to evaluate, create and sustain active living environments. - The Molecular Signatures of Exposure in Cancer Workshop, held on June 29-30, 2023, was jointly hosted by NIEHS and NCI. EGRP staff helped organize and participated in the meeting, which assessed the current state of the science of using signatures from “omic” data types to link environmental exposures to cancer and explore potential uses of such signatures of carcinogenic exposure to aid cancer prevention. Video recordings of the meeting sessions are available on the website.
- EGRP staff helped organize and participated in Unraveling Links Between Chronic Inflammation and Long COVID: Current State, Challenges, and Opportunities. The virtual workshop was hosted by the Trans-NIH Chronic Inflammation Working Group on September 19-21, 2023. The meeting agenda and recordings of the sessions are available on the workshop website.
- The planning committee for Big Data Integration: Unlocking the Potential for Enhanced Epidemiological Research included multiple staff members from EGRP. The meeting, designed to improve the use of big data for population studies by promoting an understanding of current scientific research, was held on September 27-28, 2023.
- EGRP hosted the 2023 NCI Cohort Consortium Annual Meeting virtually and in person on October 11-13, 2023. The meeting brought together consortium researchers to discuss data sharing; variables management and interoperability in data collection; using geospatial data to assess environmental and social risk factors; and project group reports. Recordings will be available in early 2024.
- EGRP staff also organized and participated in more than 15 webinars in 2023. These webinars were part of ongoing series focused on the topics below.
More details about upcoming and past webinars are available on EGRP’s events web page. Many, but not all, EGRP-supported events are recorded. Please look for the video camera icon on our events page as an indication that a recording is available.
Updated Research Resources
EGRP supports a number of web-based research tools and grant-writing resources. Here are several resources that were updated this year:
- The Diet History Questionnaire (DHQ) III now offers a sample version of its Respondent Nutrition Report, which provides individualized nutrient-level information (e.g., total energy intake, food groups, and nutrients like vitamins and minerals) compared to the Dietary Guidelines for Americans.
- The Automated Self-Administered 24-Hour (ASA24) Dietary Assessment Tool added several new resources, including an updated Respondent Nutrition Report; codebooks for the sleep modules; and demonstration videos for reporting foods, drinks, and dietary supplements. ASA24 is a freely available web-based software tool that enables automated and self-administered 24-hour diet recalls and food records. It can be used by researchers for epidemiologic, intervention, behavioral, or clinical research.
- EGRP staff led the integration of new sleep variables
 into 36 datasets in the Catalogue of Surveillance Systems (CSS)
into 36 datasets in the Catalogue of Surveillance Systems (CSS) . The CSS was created a decade ago by the National Collaborative on Childhood Obesity Research (NCCOR), and it has become an indispensable resource for childhood obesity research, providing centralized access to over 100 relevant data sets.
. The CSS was created a decade ago by the National Collaborative on Childhood Obesity Research (NCCOR), and it has become an indispensable resource for childhood obesity research, providing centralized access to over 100 relevant data sets. - Two new versions of the Healthy Eating Index (HEI) were released in 2023: HEI–2020 for 2 years and older and HEI-Toddlers-2020 for 12 through 23 months. A National Collaborative on Childhood Obesity Research (NCCOR) webinar
 provided an overview of the two resources.
provided an overview of the two resources. - EGRP created a guide to genomic summary results (GSR) that includes definitions, advice on where to find or deposit GSR data, and links to the NIH policy for sharing GSR. There has been a large increase in the number of cancer genome-wide association study (GWAS) summary statistics shared in the GWAS catalogue
 , from an estimated 7.5% of cancer GWAS studies in 2021 to 36% in 2023.
, from an estimated 7.5% of cancer GWAS studies in 2021 to 36% in 2023. - EGRP developed a web page on NIH Data Management and Sharing Plans, which includes an overview of the NIH Policy for Data Management and Sharing (DMS), how to prepare DMS plans (including for research subject to the NIH Genomic Data Sharing Policy), how to request and justify related costs, and links to more information. A new page on Biospecimen Management and Sharing provides information on suggested elements to be included in the Resource Sharing Plan section of the grant application.
- The Cancer Epidemiology Descriptive Cohort Database (CEDCD), which contains descriptive information about 64 cancer epidemiology cohort studies, is in the process of being updated. Investigators are encouraged to update their cohort data to provide the scientific community with the most up-to-date information about their studies. As of the end of 2023, 48 of the 64 cohorts in the CEDCD have updated their information.
Additional Funding Opportunities
- Innovative Molecular Analysis Technologies (IMAT) Program
- RFA-CA-24-008, Innovative Molecular and Cellular Analysis Technologies for Basic and Clinical Cancer Research (R61, Clinical Trial Not Allowed)
- RFA-CA-24-009, Advanced Development and Validation of Emerging Molecular and Cellular Analysis Technologies for Basic and Clinical Cancer Research (R33, Clinical Trial Not Allowed)
- RFA-CA-24-010, Innovative Biospecimen Science Technologies for Basic and Clinical Cancer Research (R61, Clinical Trial Not Allowed)
- RFA-CA-24-011, Advanced Development and Validation of Emerging Biospecimen Science Technologies for Basic and Clinical Cancer Research (R33, Clinical Trial Not Allowed)
- Revision Applications for Incorporation of Novel NCI-Supported Technology to Accelerate Cancer Research (IMAT Program)
- RFA-CA-24-012 (R01, Clinical Trial Optional)
- RFA-CA-24-013 (U01, Clinical Trial Optional)
- RFA-CA-24-014 (P50, Clinical Trial Optional)
- RFA-CA-24-015 (P30, Clinical Trial Optional)
- Informatics Technologies for Cancer Research (ITCR) Program
- RFA-CA-24-016, Algorithm Development (R21, Clinical Trial Optional)
- RFA-CA-24-017, Prototyping and Hardening (U01, Clinical Trial Optional)
- RFA-CA-24-018, Enhancement and Dissemination (U24, Clinical Trial Optional)
- RFA-CA-24-019, Sustainment (U24, Clinical Trial Optional)
- PAR-24-077, Addressing Health and Health Care Disparities among Sexual and Gender Minority Populations (R01, Clinical Trial Optional)
- PA-23-317, Competing Revisions to Existing NIH Single Project Research Grants and Cooperative Agreements (Clinical Trial Optional)
- NOT-HL-23-110, Notice of Special Interest (NOSI): Data Informed, Place-Based Community-Engaged Research to Advance Health Equity
- NOT-CA-24-008, Notice of Intent to Publish a Funding Opportunity Announcement for Cancer Health Disparities and Minority Health (U54, Clinical Trial Optional)
- NOT-CA-24-018, Notice of NCI's participation in PAR-23-301, Developing novel theory and methods for understanding the genetic architecture of complex human traits (R21, Clinical Trial Not Allowed)
- NOT-CA-24-019, Notice of NCI's Participation in PAR-23-302, Developing Novel Theory and Methods for Understanding the Genetic Architecture of Complex Human Traits (R01, Clinical Trial Not Allowed)
Job and Training Opportunities
Research Resources
News and Blog Posts
Stay Connected
Subscribe for Updates

Subscribe
You can subscribe and unsubscribe at any time by entering your email address and selecting your preferences on the page that follows.
Need Help?
EGRP staff can answer questions on grant funding, policies, and research resources. If you do not know who to contact we will do our best to connect you with someone who can help you.
Email Us(240) 276-6730