On this page...
Membership Application Form
Membership is granted after completion and receipt of a membership application. All memberships shall be granted upon a majority vote of the Steering Committee. To apply for membership, please complete the membership application form and send it to NCICohortConsortium@mail.nih.gov. Contact Sonia Rosenfield, PhD with any questions about applying for membership.
Download the Membership Application Form [PDF - 950 KB]Membership Eligibility
The NCI Cohort Consortium has expanded member eligibility to include additional cohorts that study cancer. Cohorts meeting the following criteria are eligible to apply for voting membership in the NCI Cohort Consortium:
- Currently recruiting or completed minimum enrollment (if applicable):
- Cohorts with cancer incidence endpoints: at least 5,000 participants (enrolled or target enrollment) with baseline risk factor data.
- Cohorts with cancer-related outcomes (e.g., survival): at least 400 participants (enrolled or target enrollment) diagnosed with cancer, with baseline risk factor data.
- Willingness to share data and actively participate in Consortium activities.
Members must also abide by the NCI Cohort Consortium's Bylaws.
Current Members
The NCI Cohort Consortium membership is international in scope. It includes investigators responsible for more than 77 high-quality cohorts who are studying large and diverse populations.
The purple regions on the map below represent the locations of populations that NCI Cohort Consortium members are currently studying.
Map of NCI Cohort Consortium Membership by Continent, 2025
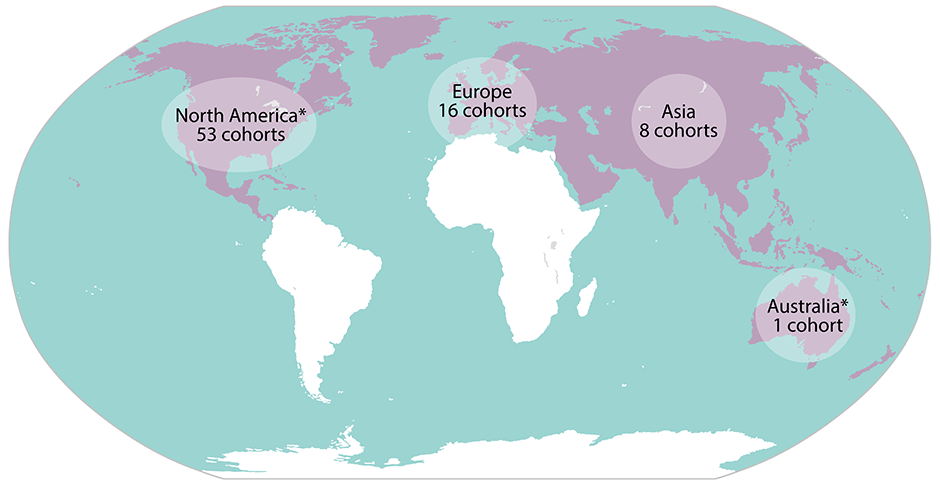
* The Breast Cancer Family Registry (BCFR) Cohort and the Colon Cancer Family Registry Cohort (CCFRC) include study participants from both North America and Australia.
Member List
A
- Agricultural Health Study
- Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study
- Arkansas Rural Community Health (ARCH)

- Atherosclerosis Risk in Communities Cohort – Cancer (ARIC-Ca)
B
- Black Women's Health Study (BWHS)
- Boston Lung Cancer Study
- Breast Cancer Now Generations Study (BGS)
- Breast Cancer Detection Demonstration Project (BCDDP) Follow-Up Study
- Breast Cancer Family Registry (BCFR) Cohort
- Breast Cancer Surveillance Consortium (BCSC)
C
- California Teachers Study (CTS)
- Canadian Partnership for Tomorrow’s Health (CanPath)
- Alberta's Tomorrow Project
- Atlantic Partnership for Tomorrow's Health (PATH)
- British Columbia Generations Project
- CARTaGENE
- Ontario Health Study
- The Manitoba Tomorrow Project
- Canadian Study of Diet, Lifestyle, and Health (CSDLH)
- Cancer Prevention Studies
- Carotene and Retinol Efficacy Trial (CARET)
- CLUE I
- CLUE II
- Cohort of Swedish Men
- ColoCare Consortium (ColoCare)
- Colon Cancer Family Registry Cohort (CCFRC)
- Connect for Cancer Prevention Study
- CONOR Cohort: General Cohort of Adults in Norway
E
- England & Wales Hodgkin Lymphoma Cohort
- European Prospective Investigation into Cancer and Nutrition (EPIC)
F
G
H
I
J
L
M
- Mayo Clinic Biobank
- Mayo Mammography Health Study (MMHS)
- Mexican American (Mano a Mano) Cohort (MAC)
- Mexican Teacher's Cohort (MTC)
- Michigan Cancer and Research on the Environment Study (MI-CARES)

- Millennium Cohort Study (MCS)
- Million Women Study
- Multiethnic Cohort Study of Diet and Cancer (MEC)
N
- Netherlands Cohort Study (NLCS)
- Northern Sweden Health and Disease Study (NSHDS)
- Nurses' Health Study I (NHS I)
- Nurses' Health Study II (NHS II)
- Nutrition Intervention Trials - Linxian
- NYU Women's Health Study (NYUWHS)
P
- Pathways Study (Pathways)
- Physicians’ Health Study I and II (PHS I & II)
- Polish - Norwegian Study PONS (PONS)
- Prostate Cancer Prevention Trial (PCPT)
- Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO)
R
S
- Selenium and Vitamin E Cancer Prevention Trial (SELECT)
- Seventh-day Adventist Cohort Study-2 (AHS-2)
- Shanghai Cohort Study (SCS)
- Shanghai Men's Health Study (SMHS)
- Shanghai Women's Health Study (SWHS)
- Singapore Chinese Health Study (SCHS)
- Sister Study (SIS)
- Southern Community Cohort Study (SCCS)
- Southern Environmental Health Study (SEHS)

- Southern Liver Health Study (STRIVE)

- Susan G. Komen Tissue Bank at the IU Simon Cancer Center (Komen Tissue Bank)
- Swedish Mammography Cohort (SMC)
- Swedish National March Cohort (SNMC)
T
- The 10,000 Families Study (10KFS)

- The Melbourne Collaborative Cohort Study (MCCS)
- The National Institutes of Health AARP Diet and Health Study (NIH-AARP)
U
V
W
Associate Member Council
The Associate Member Council (AMC) is the representative body of early career investigators within the NCI Cohort Consortium. The AMC supports the NCI Cohort Consortium mission to foster scientific collaborations of investigators engaged in large cohort studies on cancer risk and outcomes through the engagement of early career investigators and promotion of their professional development.
The overarching mission of the AMC is to engage and support early career investigators through professional development, career networking opportunities, and research collaborations within the NCI Cohort Consortium and to position members of the next generation of investigators to attain leadership roles within the Consortium. The focus of the AMC is on communication, education, training, and collaboration.
The leadership of the AMC is comprised of 4-6 early career investigators who represent the council. Membership of the AMC is voluntary and targeted to post-doctoral fellows, researchers, and early career investigators, generally within 10 years of terminal degree (e.g., PhD, MD), but is open to anyone who a) is identified as a part of the research team of participating cohorts in the NCI Cohort Consortium or b) leads epidemiologic projects based primarily on NCI Cohort Consortium data. NCI intramural investigators are eligible.
For more information or to join the NCI Cohort Consortium Associate Member Council, please email us at NCICohortConsortium@mail.nih.gov.